DRUG INTERMEDIATE OMEPRAZOLE CHLORO
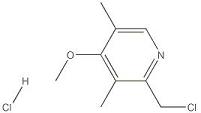
| S.No | Test | Specification |
| 1. | Description | A white to creamish white coloured crystalline powder |
| 2. | Solubility | Soluble in methylene chloride and methanol |
| 3. | Identification by IR | IR absorption spectrum of sample should be concordant with standard |
| 4. | Loss on drying (At 60°C under vacuum) | Not more than 1.0 %w/w |
| 5. | Sulphated ash | Not more than 0.5%w/w |
| 6. | Related impurities by HPLC | |
| 1. 2-Hydroxy methyl -3,5- dimethyl- 4-methoxy pyridine | Not more than 0.5% | |
| 2. 4-Hydroxy-2-chloromethyl-3,5-dimethyl pyridine HCl | Not more than 0.5% | |
| 3. Any Unknown Impurity | Not more than 0.5% | |
| 4. Total impurities | Not more than 2.0% | |
| 7. | Assay by chemical | Not less than 98.0% w/w on dried basis |
DRUG INTERMEDIATE OMEPRAZOLE SULPHIDE (UFIPRAZOLE)
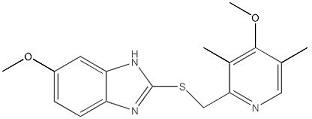
| S.No | Test | Specification |
| 1. | Description | A white to off white powder |
| 2. | Solublity | Soluble in methanol insoluble in water. |
| 3. | Identification by HPLC | The RT of the major peak in the sample chromatogram is same as that of standard chromatogram |
| 4. | Loss on drying | Not more than 1.0 %w/w |
| 5. | Melting range | Between 118°C and 125°C |
| 6. | Purity by HPLC | Not less than 99.0% w/w |